Mg Oh 2 Hcl
Hệ thống các phương trình hóa học chất hóa học đầy đủ và chi tiết nhất. Calcium hydroxide is more soluble in magnesium hydroxide therefore MgOH 2 precipitates as a solid.
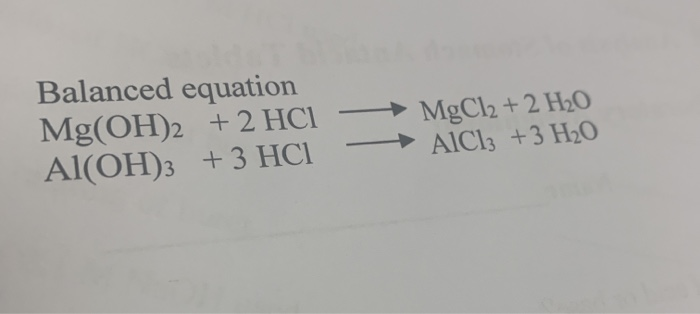
Solved Mgcl2 2 H20 Balanced Equation Mg Oh 2 2 Hcl Chegg Com
Steps Algebraic Method Balance The Equation.

Mg oh 2 hcl. 1a 0b 2c 0d Mg. Magnesium hydroxide is also avail as magnesia tabletsgenerally contain 325 mg each which can neutralize 112 meq of acid. 24305 159994 1007942 Percent composition by element.
To balance chemical equations count the number of atoms on each side of the equation and make sure the numbers of atoms on both sides of the equation are equal. Heres what I would do. MgOH2 molecular weight.
Mg OH 2 2HCl MgCl 2 2H 2 O. C_1 2 c_2 2 c_3 Mg. Balance the chemical equation algebraically.
Each milliliter is capable of neutralizing approx 27 meq of acid. Convert grams MgOH2 to moles or moles MgOH2 to grams. Chất rắn màu trắng Magie hidroxit MgOH2 tan dần.
Notice the 2-to-1 mole ratio of HCl-to-base. Starting point for our calculations. Label Each Compound With a Variable aHCl bMg OH2 cMgCl2 dH2O 2.
PT ion thu gọn là. The products from the reaction of magnesium hydroxide and hydrochloric acid are magnesium chloride and water. The balanced equation is.
MgOH 2 HCl MgCl 2 H 2 O Word equation. Since 326 g of MgOH2 neutralizes a certain amount of HCl we start with that much mass of magnesium hydroxide and determine what mass of HCl it corresponds to. HCl Mg OH2 MgCl2 H2O 1.
C_2 c_4 O. MgO H2O MgOH 2. MgOH2s 2HClaq MgCl2aq 2H2Ol for a balanced chemical equation.
Ta có phản ứng. MgOH₂ 2 HCl - MgCl₂ 2 H₂O. This comparison must be done in moles.
This reaction happens in peoples stomachs when they consume milk of magnesia in antacids to eliminate the heartburn the irritation of esophagus by the hydrochloric acid from the stomach. HCl MgOH_2 H_2O MgCl_2 Add stoichiometric coefficients c_i to the reactants and products. 0a 1b 1c 0d O.
The reaction is as follows. 030 mol Mg OH2 2 mol HCl 1 mol Mg OH2 060 mol HCl. Because MgOH 2 is listed in Table 122 Strong Acids and Bases it is a strong base.
MgOH 2 present does not automatically mean it will be used up before all of the HCl is consumed. 0a 2b 0c 1d 3. This is a limiting reagent problem.
Mg OH 2 s 2HCl aq yield MgCl2 aq 2H2O l grams HCl required 506 grams Mg OH 2 1 mol Mg OH 2 583197 grams Mg OH 2 2 mol HCl 1 mol Mg OH 2 36453 grams HCl 1 mol HCl 6326 grams HCl required Since there are only 450 grams HCl then HCl is the limiting reactant. Create a System of Equations One Per Element H. Giúp các em đạt kết quả cao trong học tập.
MgOH 2 2H 2Cl Mg 2 2Cl 2H 2 O. MgOH 2 2HCl MgCl 2 2H 2 O. It is a weak base.
2 HClaq MgOH 2 aq MgCl 2 aq 2 H 2 Ol Reaction type. Phản ứng trao đổi. 1a 2b 0c 2d Cl.
The reaction is as follows. MgOH 2 2H Mg 2 2H 2 O. 2 c_2 c_3 Since the coefficients are relative.
OH 2 184 x 103 H 2 C 8 H 4 O 4 ophthalic acid HC 8 H 4 O 4 130 x 103 H 2 C 4 H 4 O 6 tartaric acid HC 4 H 4 O 6 104 x 103 HgH 2 O 6 2 HgH 2 O 5 OH 26 x 104 SnH 2 O 3 2 SnH 2 O 2 OH 2 x 104 HCO 2 H formic acid HCO 2 19 x 104 CrH 2 O 6 3 CrH 2 O 5 OH 2 16 x 104 Hg 2 H 2 O 2 2 Hg 2 H 2 OOH 1 x 104 C 6 H 5 CO 2 H benzoic acid C 6 H 5 CO 2 646 x 105 HC 2. Magnesium hydroxide react with hydrogen chloride to produce magnesium chloride and water. 2HClaq MgOH2s 2H2Ol MgCl2aq So we use the general pathway.
To determine the amount of base in an actual tablet ideally you would dissolve it in water and titrate with acid. C_1 HCl c_2 MgOH_2 c_3 H_2O c_4 MgCl_2 Set the number of atoms in the reactants equal to the number of atoms in the products for Cl H Mg and O. 2HCl MgCl2 2H2O.
Cân bằng phương trình hóa học. Mg OH 2 2 HCl Mg 2 2 Cl 2 H 2 O. In order to find out which reactant is the limiting reagent you have to compare them to each other.
This compound is also known as Magnesium Hydroxide. C_1 2 c_4 H. This will be the.
In most titrations solutions of the acid and the base are used. Solve For All Variables a 2 b 1 c 1 d 2 4. Therefore the next step will be to.
Milk of magnesia usp is an aqueous suspension of magnesium hydroxide containing 70-85 of mgoh2. Given the amount of calcium hydroxide for the reaction to use. Magnesium chloride Water Magnesium hydroxide Hydrochloric acid Type of Chemical Reaction.
The nitrogen in C 5 H 5 N would act as a proton acceptor and therefore can be considered a base but because it does not contain an OH compound it cannot be considered a strong base. CaCO 3 2 HCl Ca 2 2 Cl CO 2 g H 2 O. Molar mass of MgOH2 5831968 gmol.
KMnO 4 HCl KCl MnCl 2 H 2 O Cl 2 K 4 FeCN 6 H 2 SO 4 H 2 O K 2 SO 4 FeSO 4 NH 4 2 SO 4 CO C 6 H 5 COOH O 2 CO 2 H 2 O. Û PT ion là. 4 rows Reaction of HCl axit clohidric react with MgOH2 magie hidroxit produce H2O nước.
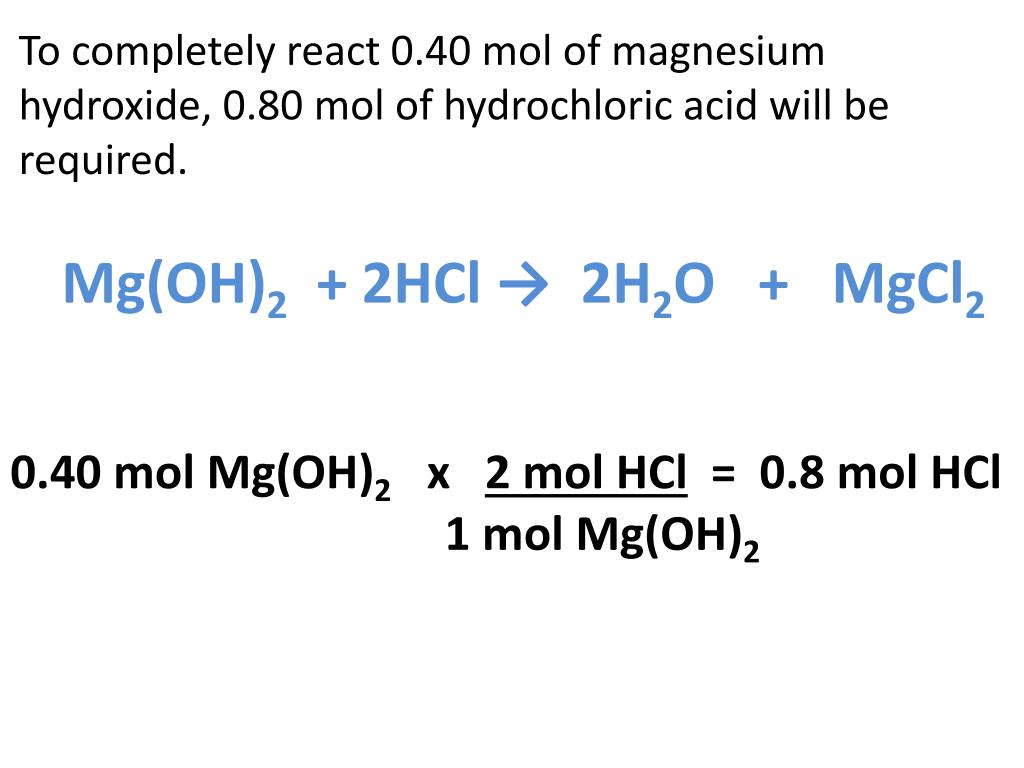
Ppt Mg Oh 2 2hcl 2h 2 O Mgcl 2 Powerpoint Presentation Free Download Id 2642841

How To Balance Mg Oh 2 Hcl Mgcl2 H2o Magnesium Hydroxide Hydrochloric Acid Youtube

Type Of Reaction For Hcl Mg Oh 2 Mgcl2 H2o Youtube

Type Of Reaction For Mgo H2o Mg Oh 2 Youtube

True Or False Mg Oh 2 Aq 3o2 G 2h2o L Mgcl2 Aq Ppt Download
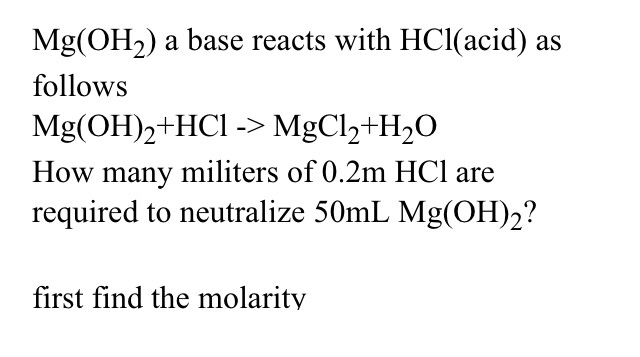
Mg Oh2 A Base Reacts With Hci A Follows Mg Oh 2 Hcl Chegg Com
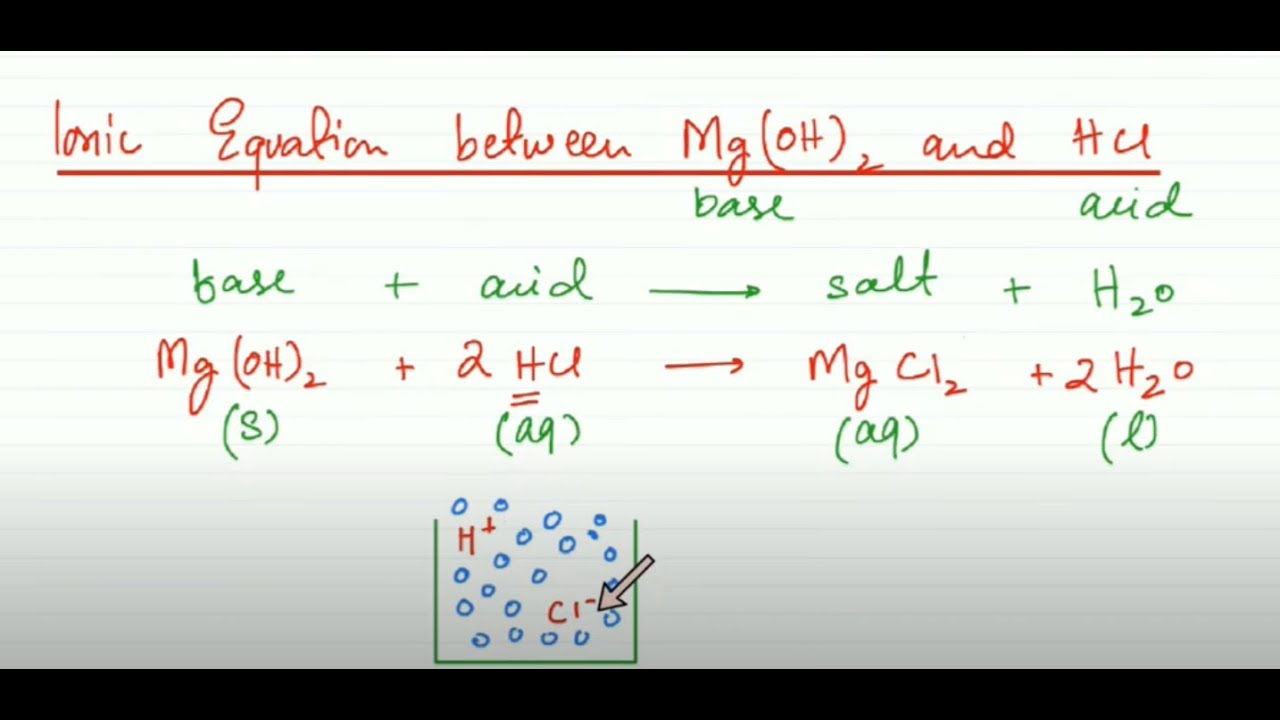
Net Ionic Equation Between Mg Oh 2 And Hcl Mega Lecture Youtube
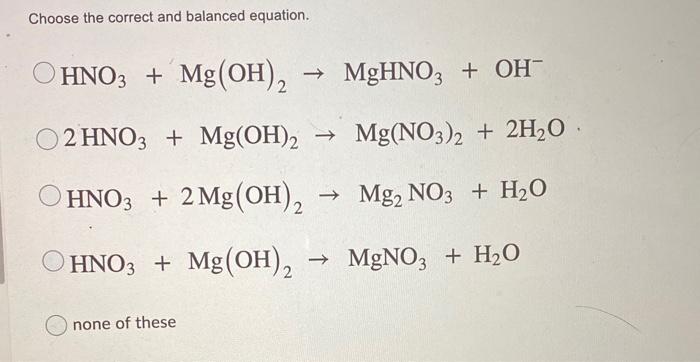
Solved Choose The Correct And Balanced Equation None Of Chegg Com

Chapter 3 4 Acids And Bases Ppt Download

Mg Oh 2 Hcl Mgcl2 H2o Balanced Equation Magnesium Hydroxide Hydrochloric Acid Balanced Equation Youtube

How To Balance Mg Oh 2 Hcl Mgcl2 H2o Magnesium Hydroxide Hydrochloric Acid Youtube

Acid Base Reactions Acids Molecules That Ionize In Water To Form Hydrogen Ions H Acids Donate Give Away Hydrogen Ions H Protons Proton Ppt Download

How To Write The Net Ionic Equation For Mg Oh 2 Hcl Mgcl2 H2o Youtube
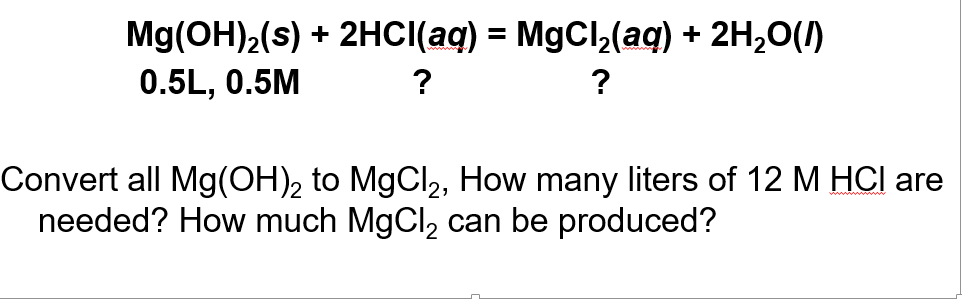
Solved Mg Oh 2 S 2hci Aq Mgcl2 Aq 2h20 0 5l 0 5m Chegg Com

How To Write The Net Ionic Equation For Mg Oh 2 Hcl Mgcl2 H2o Youtube

Type Of Reaction For Hcl Mg Oh 2 Mgcl2 H2o Youtube

Neutralization Reaction Between Mg Oh 2 S Clutch Prep
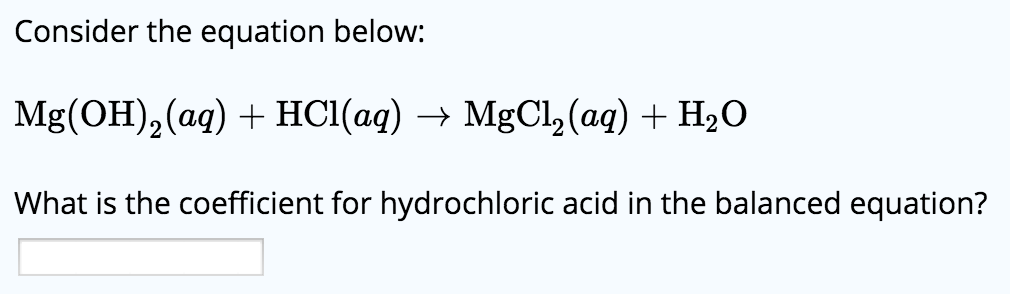
Solved Consider The Equation Below Mg Oh 2 Aq Hcl Aq Chegg Com






Posting Komentar untuk "Mg Oh 2 Hcl"